Photo by Macau Photo Agency on Unsplash
The Sinopharm vaccine forms the backbone of Namibia’s COVID-19 vaccination programme, but how much do you know about the vaccine?
(Updated on 13 July 2021 to include emergency use listing approval by the World Health Organisation (WHO) on 7 May 2021.)
On Tuesday, 16 March 2021, Namibia received a batch of 100,000 doses of the Sinopharm vaccine as a donation from China. The vaccine has been administered to hundreds of Namibians since 19 March 2021, when Namibia’s vaccination programme officially got underway.
Following are some basic facts about the Sinopharm vaccine:
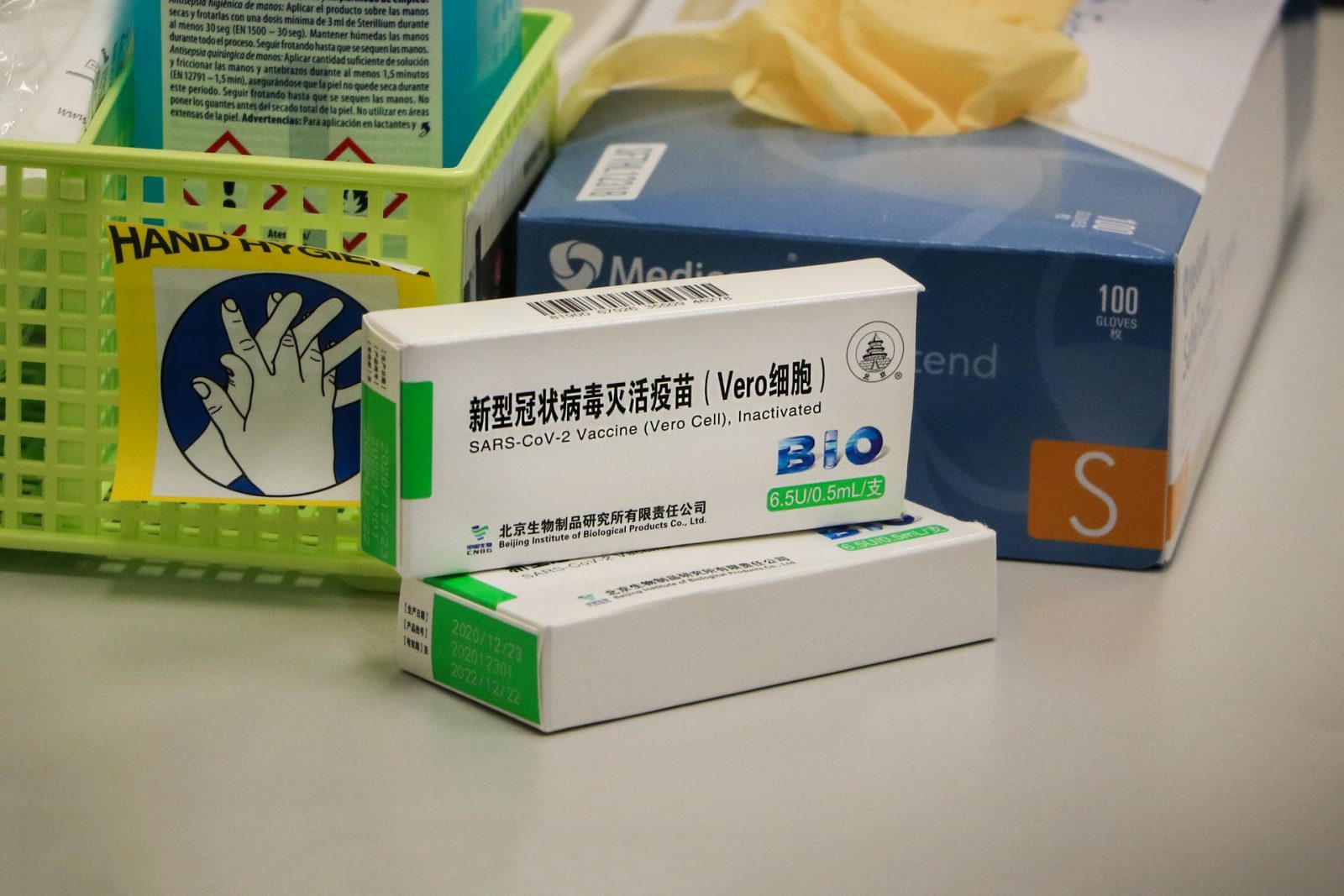
- The vaccine is produced by the Beijing Institute of Biological Products for the China National Biotec Group (CNBG), which is a subsidiary of the state-owned China National Pharmaceutical Group (Sinopharm);
- The vaccine is an inactivated or weakened virus vaccine, “which use a form of the virus that has been inactivated or weakened so it doesn’t cause disease, but still generates an immune response”;
- The vaccine is officially called BBIBP-CorV;
- The vaccine has been in emergency use since July 2020, when China launched its vaccines emergency use programme;
- September 2020 – The vaccine was granted Emergency Use Authorisation (EUA) by the Ministry of Health and Prevention (MOHAP) of the United Arab Emirates (UAE);
- 15 October 2020 – Results of phase I and II trials are published, showing the vaccine is safe for use;
- 9 December 2020 – It is reported that following a phase III trial, involving more than 30,000 volunteers, the UAE health authorities announce that the Sinopharm vaccine has an efficacy rate of 86%;
- At the end of December 2020, China’s National Medical Products Administration announced that the vaccine had officially been approved for public use. According to reports, it was also announced that the vaccine “was 79.34% effective in preventing people from developing the disease based on interim data”;
- There are currently four phase III trials, in various parts of the world, underway to establish the efficacy of the vaccine in preventing COVID-19 infection in individuals older than 18 years;
- The largest ongoing phase III trial for the vaccine is a four-country study – Bahrain, Jordan, Egypt and the United Arab Emirates (UAE) – involving 45,000 participants. The study is set to be completed between June and September 2021;
- Despite phase III trial data still being outstanding on the Sinopharm vaccine, 35 countries on four continents – including Namibia – have already approved its use to inoculate against COVID-19 infection;
- The vaccine was approved for Emergency Use Listing (EUL) by the World Health Organisation (WHO) on 7 May 2021. The EUL means that the vaccine could be distributed through the COVAX Facility to WHO member states;
For a really interesting and accessible explanation of how the Sinopharm vaccine works, view this New York Times explainer article. For more information on the vaccine from the WHO, here’s what you need to know.